Process Intensification
Increasing output of our production processes, starting by introducing an intensified Remicade process.
Click on the blocks on the left for more details.
Remicade High Titer
We are moving to a more intensive manufacturing process for Remicade, from Low Titer (LT) to High Titer (HT), increasing production with the same number of bioreactors. This is a market-driven demand. The switch to the intensified process demands installing new equipment and preparing operations for this change.
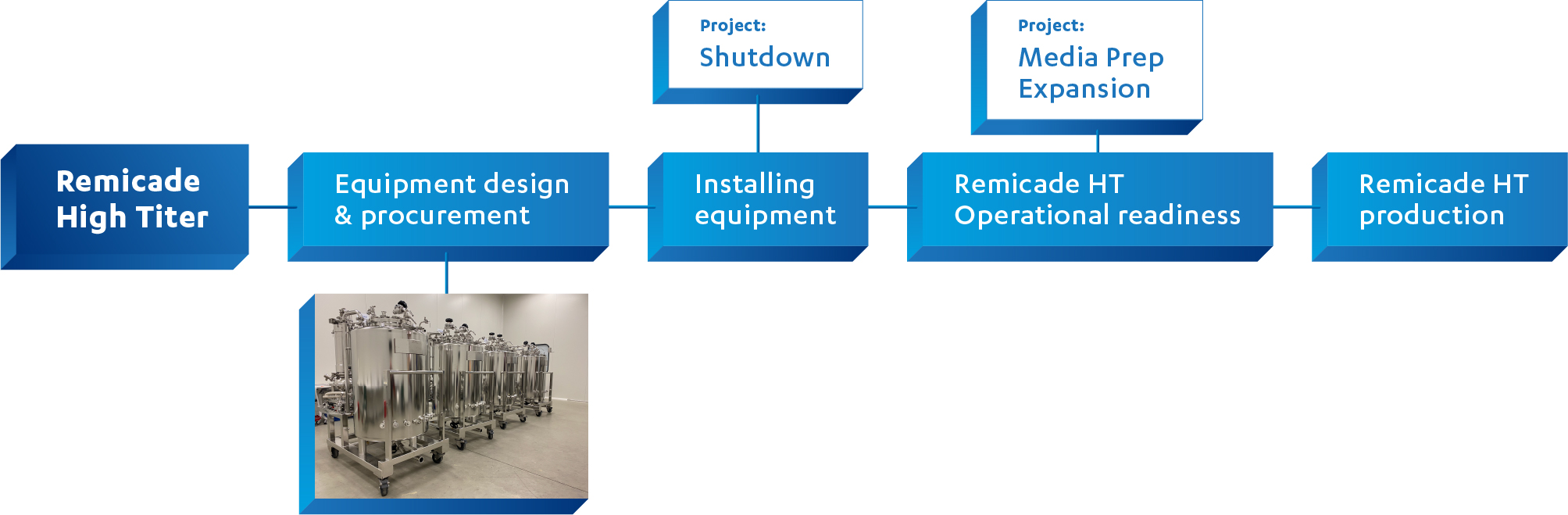
What & Where?
Preparations for the change have started in DEC22 after successful PPQ Phase, with design phase and the ordering of equipment. The shutdown in Cell Culture Suite 1 enables the transition from the production of Remicade Low Titer to Remicade High Titer, as the necessary new equipment can be adapted and installed. The Media Prep expansions then allow Remicade High Titer to be made operationally ready, since the PLC systems will be updated.
When?
The Shutdown is scheduled until end of MAR, which means that this Remicade High Titer process will start in the beginning of APR. Per 03APR24 a ramp up of the bioreactors with a start up sequence every 3 days 1 Bioreactor up 10 Hight Titer in total
Program Manager
Project Lead(s)
Vivian Kramer, Isabelle Schoeten